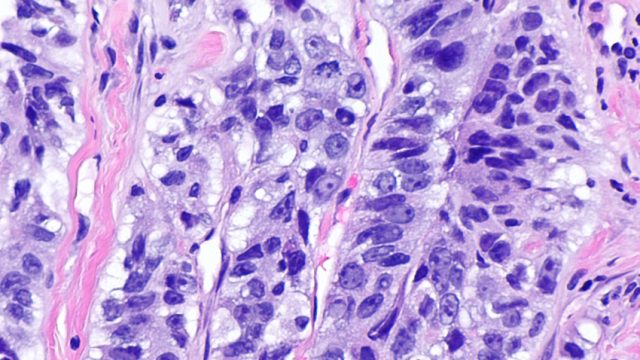
The large outer leaves of the vegetables were “literally riddled with holes, more than half their substance being eaten away.” With each step he took around the ravaged cabbages, tiny swarms of little ash-gray moths rose from the ground and flitted away. This was, it appears, the first record in the United States of the diamondback moth, an invasive pest that in its larval form shows a fondness for cruciferous vegetables. By the late 1800s the moths were chewing through the leaves of cabbages, brussels sprouts, collards, and kale from Florida to Colorado.
To fight this invasion, farmers started bombarding their fields with primitive pesticides. This worked. Or seemed to. It killed most of the moths, but those that survived the poison reproduced, and the population bounced back stronger than ever. For decades, one pesticide after another failed as the moths evolved to withstand it. Even the grievously toxic DDT was no match for the diamondback. Beginning in the late 1950s, agriculture experts started to abandon the idea of eradication and adopted a new strategy. Farmers would leave the moths alone until their numbers exceeded a certain threshold, and only then would they deploy pesticides. Remarkably, this helped. The moths did not die out, but the pest could be managed and crop damage held in check.
When Robert Gatenby heard this history of the diamondback moth in 2008, he immediately latched onto it. Gatenby is not a farmer nor an agronomist nor a fan of cruciferous vegetables—in fact, he deeply loathes brussels sprouts. He is a radiologist by training and heads the radiology department at the H. Lee Moffitt Cancer Center in Tampa, Florida. But unlike your typical doctor, he is also obsessed with the evolutionary principles put forth more than 150 years ago by Charles Darwin. The story of the diamondback moth appealed to Gatenby as a useful metaphor for his own project—one concerned not with crops but with cancer.
Like the diamondback moth, cancer cells develop resistance to the powerful chemicals deployed to destroy them. Even if cancer therapies kill most of the cells they target, a small subset can survive, largely thanks to genetic changes that render them resistant. In advanced-stage cancer, it’s generally a matter of when, not if, the pugnacious surviving cells will become an unstoppable force. Gatenby thought this deadly outcome might be prevented. His idea was to expose a tumor to medication intermittently, rather than in a constant assault, thereby reducing the pressure on its cells to evolve resistance.
Just as ecologists allow for a manageable population of diamondback moths to exist, Gatenby’s method would permit cancer to remain in the body as long as it doesn’t spread further. To test this idea, Gatenby got permission in 2014 to run a trial on advanced-stage prostate cancer patients at Moffitt. The patients had cancer that no longer responded to treatment; their drug-resistant cells were winning an evolutionary battle within the body, surviving an onslaught of toxic drugs where weaker cancerous cells had succumbed. The hope was that, by using a precise drug-dosing scheme developed using evolutionary principles, they could slow the rise of the mutations that would endow some cancer cells with the fitness to survive. Gatenby's name for the approach was adaptive therapy.
One of the patients in the trial was Robert Butler, a British oil-exploration engineer who had retired in Tampa. In 2007 he was diagnosed with prostate cancer, and seven years later, after taking the drug Lupron and getting blasts of radiation, his prostate tumor had progressed to stage 4, advanced cancer. Butler did not give up, though. He tried a newly approved immunotherapy treatment—one that involved having cells from his blood sent by courier to a facility outside Atlanta, where they were mixed with a molecule that activates immune cells, and then shipped back to Florida to be injected back into him. The treatment was expensive—its sticker price can be as high as $120,000—but the threat that the cancer would progress remained.
When Butler and his wife showed up at his oncologist’s office at the Moffitt Cancer Center in August 2014, they braced for what would come next; they had heard about invasive treatments, like radioactive seed implants. So they were intrigued when the doctor told them about Gatenby’s trial and asked if Butler wanted to participate. He would take a powerful and exceedingly expensive drug called Zytiga, but not in the scorched-earth, kill-all-the-cells fashion that is standard. Instead he would receive only as much Zytiga as was necessary to stop the cancer from growing. The idea was radical and counterintuitive. His last best shot at escaping death from his cancer was to give up on curing it.
Knowing the modified Zytiga regimen wasn’t designed to rid him of cancer left Butler, the engineer, with a question about how the doctors would measure the success of their new treatment approach. He asked, “How do we know this stuff is working?” And one of his doctors replied, “Well, you won’t be dead.”
In the United States, we use military metaphors when we talk about cancer. We battle and we fight, and if we survive, we’re victorious. The attitude traces back in part to 1969, when the Citizens Committee for the Conquest of Cancer ran an ad in The Washington Post and The New York Timesimploring the president with the words “Mr. Nixon: You can cure cancer.” The call to action helped trigger the country’s “war on cancer” with a determination that, using enough medical weaponry, the malignant foe could be obliterated.
By the mid-1970s, however, signs were beginning to emerge that certain strategies aimed at total eradication were liable to backfire. Against this backdrop, a cancer researcher named Peter Nowell published a seminal paper in Science in 1976. Nowell conjectured that evolutionary forces drive certain cell populations in tumors to become progressively more malignant over time. The cells inside a tumor are in competition, not only with nearby healthy cells, Nowell argued, but also with each other. Nowell suggested—and later research confirmed—that certain DNA alterations grant cancer cells resistance against chemotherapy or other treatments, causing them to edge out drug-sensitive cells through a process of natural selection.
Nowell conveyed his ideas to his students at the University of Pennsylvania School of Medicine, sometimes smoking a cigarette as he lectured. His theories were respected but slow to catch on. He emphasized that tumors may become deadlier as they accumulate more genetic errors. It was an idea ahead of its time. Scientists back then didn’t have the technical capability to measure all the changes in the vast genomes of tumor cells. Instead, they could sequence only little tidbits of DNA at a time, and most scientists viewed cancers as the fruit of just a few genetic mutations.
One of the medical students listening to Nowell lecture in the late 1970s happened to be a young Bob Gatenby. But Nowell’s ideas didn’t make a strong impression on him, Gatenby says; instead, what inspired him was what he witnessed in his first years as a practicing radiologist on the bloody front lines of the war on cancer.
By the mid-1980s, Gatenby had secured a job at the Fox Chase Cancer Center in Philadelphia. At that hospital and others around the country, clinical trials were putting breast cancer patients through an extreme treatment: a combination of a potentially lethal dose of chemotherapy followed by a bone marrow transplant. The treatment was harrowing. The women had diarrhea and nausea, and some had so much lung damage they had difficulty breathing. Others experienced liver damage and weakened immune systems that left them vulnerable to serious infections. As a radiologist, Gatenby’s job was to interpret x-rays and other scans of the patients, and he saw the treatment failing. Out of more than 30,000 women with breast cancer in the US who underwent the procedure between 1985 and 1998, as many as 15 percent died from the treatment itself. “What happened was these women suffered horribly, and they weren’t cured,” Gatenby says.
Around the same time as the breast cancer trials, the father of a colleague of Gatenby’s came to the hospital to receive an initial, aggressive round of chemotherapy for lung cancer. According to the colleague, her father arrived on a Friday with no apparent symptoms and was dead by Monday. “That event to me was very traumatizing,” Gatenby recalls, and the cause to him seemed obvious. “I couldn’t understand why you would treat someone with a fatal disease and kill them with your therapy. It just didn’t feel right to me.” During this fraught period, Gatenby’s own father died from esophageal cancer.
Gatenby felt there must be a better way to treat cancer—to outsmart it rather than carpet-bomb it. He had studied physics in college and believed that biologists could leverage equations to capture the forces driving cancer the same way physicists use math to describe phenomena like gravity. Whereas Nowell had put forth general theories about how cancers followed evolutionary principles, Gatenby was taking a further leap: He wanted to figure out a way to describe the evolution of cancers with mathematical formulas.
By 1989, Bob Gatenby was preoccupied with modeling the evolution of cancers. During the day he would scrutinize the x-rays of cancer patients, and at night, after he and his wife had put their young kids to bed, he would sit at the kitchen table in their suburban Philadelphia home and pore over medical journals. The patterns he started seeing in the literature led him to a question: What if cancer cells outcompete normal, healthy cells in the body in the same way an animal species edges out its competitors in nature?
Gatenby recalled that ecologists had come up with equations to describe the balance between predators and prey. As an undergraduate at Princeton University, he had learned the classic example of the math that plotted how growing populations of snowshoe hares fuel the rise of the lynx that feed on them. He began dusting off old books and buying new ones to educate himself on species interactions.
For a year Gatenby read and mulled. Then, in 1990, on a family trip to the Atlantic coast of Georgia, he found himself stuck in a hotel room one afternoon with his two napping children. Out of nowhere, an idea presented itself. He grabbed a pad of hotel stationery and a pen and began scribbling down some key formulas from population ecology. Those formulas, called Lotka-Volterra equations, have been used since the 1920s to model predator-prey interactions and, later, competition dynamics between species, and were among the ones he had recently brushed up on at home. Gatenby thought this set of formulas could also describe how tumor cells compete with healthy cells for energy resources such as the glucose that fuels them.
When he returned to Philadelphia, he spent what time he could at a typewriter composing a paper that laid out this theoretical model. As soon as he finished, he showed it to some colleagues. He didn’t get the response he had hoped for: They thought it was ridiculous to try to use ecological equations to model cancer. “To say that they hated it would not do justice to how negative they were about it,” he says. His peers thought that a brief set of formulas couldn’t capture cancer’s seemingly infinite complexities.
Louis Weiner, who worked alongside Gatenby at the time, recalls that their colleagues viewed Gatenby’s ideas as offbeat. “Treatment orthodoxy at that time favored high-intensity, dose-dense treatments aiming to eradicate every last tumor cell in a cancer patient,” says Weiner, who is now director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, DC. “Bob’s perspective was antithetical to those beliefs.”
But Gatenby pressed on and succeeded in getting the paper, chock-full of Lotka-Volterra equations, accepted in the prominent journal Cancer Research in 1991.
Despite the publication of his theory, he still couldn’t convince oncologists that his idea had practical merit. “I think that they felt intimidated,” Gatenby says. “Most physicians are mathematically illiterate.” He found that the medical establishment was reluctant to publish much of his follow-up work.
In the years afterward, Gatenby moved up the ladder to lead the department of diagnostic imaging at Fox Chase Cancer Center. He was later appointed head of the department of radiology at the University of Arizona College of Medicine in Tucson, and he continued to garner recognition for his skilled interpretation of scans and to receive federal grants to study cancer.
Then, in 2007, the Moffitt Cancer Center offered Gatenby a job as chair of the radiology department. He had a condition: He would come if the hospital created a division where he could pursue in earnest the link between Darwin’s principles and cancer. The Integrated Mathematical Oncology Department, born from this negotiation, is the first math department in a cancer hospital, he says. Finally, Gatenby had a place where he could put his ideas to the test.
Gatenby arrives at his corner office at Moffitt most days by 7 am. He’s 67 now, and his hair is gray at the temples, but his eyebrows are still brown. His children—the ones who were napping in that hotel room when he jotted down his Darwinian inspiration—now have children of their own, and he has the “I ♥ Grandpa” coffee mug to prove it. A hospital lanyard around his neck, he rolls up his crisp shirtsleeves and settles down at his desk. Outside his office, roughly 30 scientists and PhD students spend their days researching patterns of cancer growth using equations like those describing population dynamics.
To Gatenby's knowledge, no one had endeavored to exploit evolution against cancer in a clinical trial until he developed his prostate cancer experiment. He picked prostate cancer to test this approach partly because, unlike other cancers, a routine blood draw for a molecule called prostate-specific antigen (PSA) can offer an immediate proxy for the cancer’s progression.
To design a clinical trial, Gatenby and his Moffitt collaborators first needed to account for their idea that tumor cells vie against each other for resources. They turned to game theory to plot this dynamic and plugged the numbers into the Lotka-Volterra equations. The computer simulations they ran with these equations estimated how quickly drug-resistant cells would outcompete other tumor cells when exposed to the continuous dosage of Zytiga typically given to advanced-stage prostate cancer patients.
In the simulations, the typical administration of the drug led to drug-resistant cancer cells rapidly running rampant. The treatment would ultimately fail each time. That bleak outcome matched up with the results seen in hospital records. In contrast, the computer simulations suggested that if Zytiga were administered only when the tumor seemed to be growing, then the drug-resistant cells would take much longer to gain enough advantage to overrun the cancer.
In 2014 the Moffitt team managed to get the first small study to test this adaptive therapy approach off the ground, recruiting Robert Butler and a small group of other men with advanced prostate cancer. Butler’s oncologist explained to him how it would work. He would remain on the Lupron he’d taken for years, and each month he would go to the hospital to get his PSA level tested, to judge whether his prostate tumor was growing. Every three months, he would get a CT scan and a full-body bone scan to watch for disease spread. Whenever his PSA level edged above where it stood when he entered the trial, he would start taking the more powerful Zytiga. But when his PSA level fell to under half of the baseline, he could go without Zytiga. This is appealing because Zytiga and drugs like it can cause side effects like hot flashes, muscle pain, and hypertension.
The Moffitt approach also promised to be far cheaper than taking Zytiga continuously. When purchased wholesale, a one-month supply costs almost $11,000. Butler had health insurance, but even so, his first month’s supply each year would set him back $2,700 in out-of-pocket copayments, and $400 a month thereafter. Going off the drug whenever his PSA level was low would translate to huge cost savings.
Butler was participating in a so-called pilot trial, which was less rigorous than a large clinical trial, because it didn’t randomly assign patients to receive the experimental or standard treatments. Rather, the study relied on a group of patients treated outside the trial as well as results from a 2013 paper on Zytiga to come up with a benchmark for how patients typically fare when receiving continuous dosing of the drug.
When the early results of their new trial trickled in, the Moffitt scientists were gratified and relieved. Ahead of the trial, “we were, to be honest, terrified,” Gatenby says. The benefit of adaptive therapy appeared to be huge. Of the 11 men in the study, one left the trial after his disease spread, but most were living longer than expected without their cancer progressing. Men getting continuous dosing of Zytiga go a median of 16.5 months before the cancer becomes resistant to the drug and spreads. In comparison, the median time to progression for the men receiving adaptive therapy was at least 27 months. Moreover, they were on average using less than half of the standard amount of Zytiga. Joel Brown, an evolutionary ecologist and one of Gatenby's collaborators, said the team felt a moral obligation to get the word out: “The effect was so big that it would be unethical not to report it immediately,” he says.
They published a report in 2017, far earlier than anticipated, to a generally positive reaction from prostate experts—particularly because it suggested a way that people with cancer might live longer with less medication. “If you can reduce side effects, I think that’s fantastic,” says Peter Nelson, an oncologist who studies prostate cancer at the Fred Hutchinson Cancer Research Center in Seattle. “Conceptually it’s a beautifully simple approach.” Jason Somarelli, a biologist at the Duke Cancer Institute, calls Gatenby a pioneer: “He’s turning cancer into a chronic disease.”
Butler, who is 75, has gone for long periods off Zytiga—with stretches lasting as long as five months. “I’m now the poster boy, they say,” Butler says. He’s one of the best responders in the study.
Some doctors are already trying adaptive therapy on patients outside of clinical trials. In 2017 a doctor in Oregon, inspired by Gatenby’s pilot study, started a prostate cancer patient on a modified version of the approach when he refused the standard continuous dosing. She has since started treating a second man using adaptive therapy. Other oncologists might be doing the same. It’s nearly impossible to know for sure, because adaptive therapy doesn’t require government approval. The protocol uses already-approved medications, and the US Food and Drug Administrationdoesn’t police specific dosing schedules.
Experts urge caution, however. The prostate cancer study was very small, and without a randomly assigned control group the results aren’t truly reliable. While the majority of the men in the trial remain stable, four more saw their cancer progress since the paper came out. “This is an approach that now needs to be carefully studied in prospective clinical trials before it is adopted into clinical practice,” says Richard L. Schilsky, chief medical officer for the American Society of Clinical Oncology. Years could pass before a large-scale test of adaptive therapy takes place. Len Lichtenfeld, interim chief medical officer of the American Cancer Society, echoes Schilsky’s concerns. “Is it intriguing? Yes,” Lichtenfeld says. “But there is still a long way to go.”
Gatenby agrees that adaptive therapy needs rigorous testing. He conveys a kind of humility you don’t see very often in the upper reaches of medical science. He told me multiple times that he is not an interesting subject to write about, and more than once I heard close colleagues mangle the pronunciation of his name (which is pronounced GATE-en-bee); apparently he had never corrected them. But when he believes in something, he doesn’t relent. And he believes in adaptive therapy. “He’s like a teddy bear, but underneath that soft exterior he’s made of steel,” says Athena Aktipis, who studies theoretical and cancer biology at Arizona State University and has collaborated with Gatenby.
Late last year, Gatenby presented his work at a meeting of prostate cancer specialists. In the question and answer session afterward, an attendee shared his surprise at the results. “I guess what you’re saying is that we’ve been doing it wrong all these years,” the man mused, according to Gatenby. “I was literally speechless for a few moments,” Gatenby admits, “and then I said, ‘Well, yeah, I guess that’s what I’m saying.’” He is still dwelling on the exchange and wishes he could somehow find the man and apologize. He’s not taking back what he said; he does think the profession can do better. But, he says, “I should have been more diplomatic.”
In 2016, a couple dozen researchers gathered in a conference room at an ultramodern genetic sequencing center along the banks of the River Cam, 9 miles outside of Cambridge, England. The gathering brought together experts to discuss how principles of ecology might apply to cancer. When they took a break, their idea of fun was to play a round of “Game of Clones,” in which a small group of scientists pretended to be cancer cells trying to persuade the maximal number of other researchers bouncing around the room to be their malignant clones.
During this meeting, one overarching theme kept popping up: Evolution doesn’t operate the same way within all cancers. It’s not even clear that Darwinian natural selection always determines the genetic mutations that abound within a tumor. A study of colon cancer samples conducted by one of the conference attendees, Andrea Sottoriva of the Institute of Cancer Research in London, and Christina Curtis, a computational biologist at Stanford University, suggested a different pattern.
When colorectal tumors begin to form, there seems to be a “big bang” of mutations. This initial explosion of cellular diversity in these colon cancers seems to be followed by a period in which random genetic changes arise and become more prevalent out of pure happenstance rather than because the mutations confer some sort of competitive advantage. It’s still unclear whether adaptive therapy, which operates on the assumption that there’s Darwinian competition between tumor cells, would work well for cancers where the mutations arise continuously by chance.
Still, a kind of consensus emerged, and a year after the Cambridge meeting, the organizers published a statement outlining how cancers might be better classified. Twenty-two researchers—some of the biggest names in the field of evolutionary oncology, including Gatenby—coauthored the document.
One important factor in the group’s suggested classification scheme is a measure of how swiftly a cancer is mutating. In the past decade, faster DNA sequencing tools have shown that Nowell—Gatenby’s old professor, the cigarette-smoking pioneer in applying evolutionary thinking to cancer—was prescient: Individual tumors often bristle with rapid-fire genetic changes. Rather than two or three initial errors setting off a chain of uncontrolled growth, many tumors are the result of several series of mutations. A significant experiment published in 2012 found at least 128 different DNA mutations in various kidney tumor samples from one patient, for instance. There's some evidence that the more mutations there are, the more aggressive a cancer tends to be, suggesting a higher chance that one of these DNA changes will confer tumor cells with the potential to be drug-resistant. Given technological advances, it’s not too far-fetched to think that within the coming decade, doctors will routinely measure the amount of mutations in their patients’ tumors.
Today most cancers are assessed using a system that dates back to the 1940s. Doctors typically evaluate factors such as whether a cancer has spread to lymph nodes or beyond and on the basis of these attributes determine its “stage.” On one end of the spectrum are stage 1 cancers, which are relatively confined, while at the other end are stage 4 cancers, which have spread extensively. Crucially, this system of assigning cancer a stage doesn’t formally take a cancer’s genetic mutations into account.
The suggested categorization system that grew out of the Cambridge meeting would look at cancer in a completely new way. Rather than four stages of cancer, the authors of the 2017 consensus statement propose no less than 16 different categories—for example, tumors that have slow cell turnover and a low rate of accumulating mutations, or tumors that are a hotbed of genetic diversity with quickly replicating cells competing for resources. This latter type of tumor might be the most likely to evolve a way to outcompete drug-sensitive cells in the body and thereby could, in some cases, be the most dangerous. A fast-moving cancer of this kind might also be the best candidate for adaptive therapy.
Around the time the consensus statement came out, Gatenby and his collaborators in Tampa were hard at work running cell experiments in a lab down the hall from his office. The goal was to prove a key tenet of adaptive therapy. Gatenby’s approach assumes that when treatment is removed, drug-resistant cancer cells will replicate more slowly than drug-sensitive cells. The theory rests on the assumption that those resistant cells need lots of energy to maintain their armor against the medication meant to kill them. During treatment breaks, the thinking goes, the fuel-hungry resistant cells are outcompeted by drug-sensitive cells, which need fewer resources to thrive.
Granted, this experiment happened in a petri dish, not a human body—or even the body of a lab rat. Some leading cancer specialists agree with Gatenby that drug-resistant cells are likely outcompeted by other cells when cancer medication is withdrawn. But, say others, what if Gatenby is wrong? What if resistant cells actually thrive during the period when the patient is taken off drugs? The risks are high. No one wants to hasten death.
Rethinking cancer as a chronic illness requires a mental shift—a shift that other changes in cancer therapy might be easing. There’s a practice of letting cancer patients take doctor-supervised “drug holidays” from their medications, for instance. And we’ve adapted our thinking when it comes to medicine before. Doctors once thought that stress was the primary culprit behind ulcers, but biologists uncovered a bacterium as the main cause. More recently we’ve gotten used to the weird idea that trillions of bacteria live in our gut microbiome.
Perhaps, then, it isn’t a huge stretch to think we might tolerate coexisting with cancer cells as long as we can prevent them from growing unchecked. Whereas Darwin put forth ideas about what has become known as macroevolution—the rise and fall of species, whether they be beetles or bald eagles—this new view of cancer could be an example of what we might call “endo-evolution”: natural selection playing out within an organism’s own tissues.
The American Cancer Society acknowledges that some cancers are already managed as chronic illnesses. In certain cases, doctors simply try to keep the malignancies from spreading with new rounds of medication. Gatenby’s adaptive therapy aims to take the guesswork out of the treatment. More trials at Moffitt are in the planning stages or underway for cancers affecting the breast, skin and thyroid, in addition to a new, bigger trial in prostate cancer patients. Across the country, in Arizona, Athena Aktipis and her husband and scientific collaborator, Carlo Maley, have secured a grant to begin a breast cancer trial using adaptive therapy in conjunction with a local branch of the Mayo Clinic.
But the idea of cancer as an implacable enemy that needs to be annihilated runs deep. Even Gatenby feels it, particularly when it comes to children. When his daughter was a teenager, one of her classmates died from a form of cancer called rhabdomyosarcoma. He never met his daughter’s friend but heard about his decline. Then, last year, a pediatric oncologist at Moffitt approached him to see if therapy inspired by evolutionary theory might work to fully weed out cancer from children newly diagnosed with that same disease. In the highest-risk group, that cancer kills as many as 80 percent of patients within five years.
In October, they met to begin designing a study. This trial will use a more sophisticated evolutionary model to cycle patients on and off of several drugs. The hope is to deploy the additional drugs to kick the cancer while it’s down, and thereby drive it to extinction. It’s an ambitious goal.
For now, Gatenby is most focused on managing advanced cancers in adults, and doing so as a chronic disease. In that sense, he’s challenging the words emblazoned on the outside wall of the Moffitt Cancer Center: “To contribute to the prevention and cure of cancer.” Robert Butler has pondered these words too, which he passes when walking into the building for checkups and treatments. “Certainly, in my case there’s no intention of cure. What we’re doing is control. So that’s not really the correct logo anymore, is it?” he says. Butler tells me about a time when he and some of the Moffitt researchers brainstormed alternative slogans. “We finally came up with ‘Our aim is to make you die of something else’—which I thought was lovely,” he adds. “It’s more true.”
Source: WIRED, Full Article