Research Proposal
Prepared by: Tommy Rodriguez, M.S. for Pangaea Biosciences
Premise: This research proposal seeks to investigate the possibility that naturally occurring radiation (background radiation) could alter species composition (genotype → phenotype) over extended timeframes. We look to establish a parallel between changed levels of natural radiation and significant evolutionary events. In particular, we will draw our attention to the establishment of a modern atmosphere and the changes in radiation levels that came thereafter. Our study will focus less on early microbial life, and more on modern vertebrates/invertebrates and the subsequent colonization of land species that took place after the ozone layer was established. By implementing a reliable framework in which to test and evaluate genetic variance over successive generations, we will demonstrate that naturally occurring radiation is more influential in creating genetic variance than previously thought. In doing so, we hope to create a standard model for determining expectancies in population genetics based on exposure to multiple levels of radiation.
Aim: Although the effects of natural radiation on the cell biology of individual organisms have been widely investigated, not enough research has been done to determine whether naturally occurring radiation has serious precedence over biological evolution, past and present. While a handful of studies have touched upon the development of early microbial life, there has been a dearth of publications on the role of background radiation on ecosystems as a whole. For purposes of this study, we will draw our attention on two forms UV radiation: UVA, & UVB. UV radiation is found in sunlight and it ionizes at about 120 nm to 10 nm.
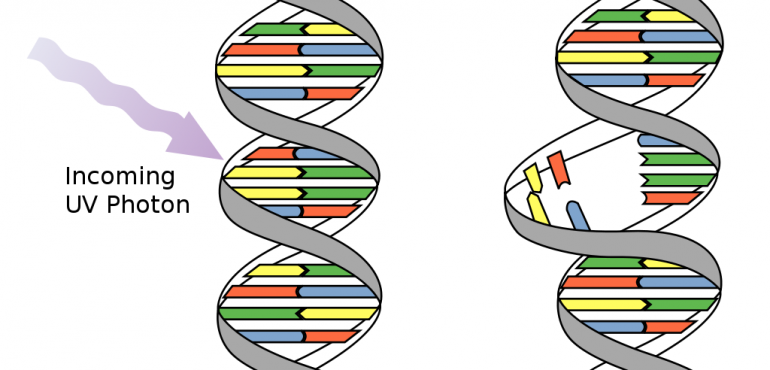
Quite simply, here is what we know about the biological effects of natural radiation: The sun emits short-wave UV radiation that is potentially harmful to life. UV radiation can disrupt the normal chemical processes of cells, causing them to become damaged, to grow abnormally or to die. Furthermore, ionized radiation can create mutation in DNA or RNA genomes. In particular, two nucleotide bases in DNA – cytosine and thymine – are most vulnerable to the effects of radiation. For low levels of radiation exposure (or non-ionized radiation exposure), the biological effects are so small they are thought to cause little or no damage to cells.
Genetic mutation and inheritance are not mutually exclusive. Evolution acts upon genetic mutation to create variance within populations of organisms, over successive generations. Needless to say, because genetic mutation is the raw material needed for biological evolution to occur, it seems almost fitting to propose the following questions:
- What role, if any, does naturally occurring radiation (background radiation) – both cosmic and terrestrial – play in shaping the outcome of living systems over the course of time?
- Can we attribute significant evolutionary events in Earth’s history to radioactive events of cosmic or terrestrial proportions?
- Moreover, is it possible to quantify the amount of genetic variation over successive generations in groups of organisms that are directly exposed to intense doses of low level (UV) radiation versus those that are non-exposed?
To what extent does the germline become affected by natural radiation is subject to debate and it should be explored further. Some might argue that timescale limitations coupled with the rarity of mutation [as passed from one generation to the next] would make such an experiment impractical in laboratory settings. We, however, would kindly disagree with that assessment. Despite a unique set of challenges involved in such an experiment, we believe to have developed a proper framework in which to detect, assess, and quantify the slightest biological change over successive generations; leading us to reliable conclusions about the long term effects of naturally occurring radiation on living systems. Our study will depend heavily on the sensitivity of genetic analysis (comparative genetics) to determine tiny amounts of genetic disparity within a test population over successive generations. This experiment will also rely exclusively on model organisms that have adjusted their sexual reproductive patterns to satisfy remarkably short life cycles. Our laboratory enclosures will be designed to mimic a number of different natural environments found on Earth; including aquatic environments, semi-aquatic environments, temperate environments, and dry environments. Other conditional variables will be introduced on occasion, as needed. For a list of test candidates, details, and explanations, please look under “Timetables & Materials.”
Our study will consist of the following groups:
(a) Experimental Group: This group will be exposed to intense low level UV radiation (between 180 and 400 nm) over the course of many life spans. The sun is our primary natural source of UV radiation. The goal is to create conditions that are consistent with solar pattern fluctuations.
(b) Control Group: This group will remain non-exposed to intense low level UV radiation and will only receive low-frequency visible light over the course of many life spans. Protective measures will ensure that undesirable amounts of radiation do not “leak” into their environments.
And so, which set of organisms might produce the most amount of genetic variation?
Timetables & Materials: Given the large scale time periods involved with biological evolution, it is unreasonable to expect any major phenotypic changes to our model organisms throughout the course of this study. However, we would also point out that new species arise as a result of a gradual accumulation of tiny genetic mutations inherited over successive generations. Moreover, even tiny mutations could have significant consequences over the course of hundreds or thousands of generations. We should keep this mind as we go forward.
This project is expected to run three years and approximately 12–96+ generations. While these figures are just rough estimates at this point, we have determined that a three-year timeframe should produce a sufficient amount of genomic data that would allow us to effectively decipher patterns of genetic variance [to lesser and higher extents] within the gene pool of multiple organisms over successive generations.
Lastly, we have narrowed our list of model organisms to the following specimens:
I.Fruit Flies (Drosophila melanogaster)
II.Tadpole shrimp (Triops cancriformis)
III.Turquoise killifish (Nothobranchius furzeri)
IV.Laboratory Mice (Mus musculus)
Quick notes about our specimen selection:
- Fruit flies require little care, culture, and equipment and uses little space even when using large cultures, and the overall cost is low. (University of Sussex)
- Fruit flies are small and easy to grow in the laboratory and their morphology is easy to identify once they are anesthetized. (University of Sussex)
- Fruit flies have a short generation time (about 10 days at room temperature) so several generations can be studied within a few weeks. (University of Sussex)
- Fruit flies have a high fecundity where females lay up to 100 eggs per day, and perhaps 2000 in a lifetime. (University of Sussex)
- Turquoise killifish is the shortest lived vertebrate species bred in captivity. (Max Planck Institute for Biology of Ageing)
- Ageing progresses very rapidly in this fish and is characterized by cancer, pigment loss, and age-dependent deterioration in motor and learning skills. (Max Planck Institute for Biology of Ageing)
- Mice are small, have a short generation time and an accelerated lifespan, keeping the costs, space, and time required to perform research manageable. (The Jackson Laboratory)
Materials, Supplies & Budgets (based on a three-year span):
*for an estimated budget please download the full report
What We Hope to Achieve: Humans and other organisms cannot escape exposure to radiation; it is all around us in the environment. Traces of natural radiation are found in oceans and waterways, rocks and soils, plant materials and in the atmosphere surrounding our planet. Over time, life has become fairly tolerable to high levels of natural radiation, including sun-emitting UV radiation. Consequently, exposure to natural radiation also places a heavy toll on metabolism and productivity in terms of costs associated with synthesis of pigments, antioxidants, and gene-repair mechanisms. The rate and accuracy of gene-repair mechanisms have an influence over the process of evolutionary change –– and thus, determining the amount of genetic diversity that can be attributed to radiation damage will be the main theme of this investigation.
We believe this study presents several important opportunities that are worth exploring. In our efforts to better understand these natural processes and to enhance our understanding of biological evolution, we also hope to shed light on the following underlying implications:
- What correlation exists, if any, between changed levels of natural radiation and significant evolutionary events in Earth’s history?
- The thinning of the stratospheric ozone layer during the last decades has increased the levels of ultraviolet-B radiation. What does that mean for modern ecosystems and the environment?
- With an apparent rise in certain types of genetically inherited disorders, could there be a possible link between a thinning ozone layer and increased levels of ultraviolet-B radiation?
- As we speak, major space agencies worldwide are planning the colonization of Mars. In what way could exposure to high levels of solar radiation affect the first colonies of plants and animals to colonize the red planet over successive generations? Might we expect similar consequences in terms of deep space voyages requiring multiple generations of colonists?
The Need for Funding: We are committed to working side by side with academic institutions, private corporations, and government agencies on a number of Research & Development projects. Pangaea Biosciences has seen measurable success and we are now seeking to expand our Research & Development projects beyond the spectrum of computational biology. As a start-up firm, we lack the financial resources of bigger institutions. Thus, we seek funding from outside sources if our research is to remain sustainable.
Pangaea Biosciences is pleased to present this proposal for your review. Our proposal requests funding to help us obtain the proper resources needed to embark on this study. We hope you will consider funding all or part of our request. Any help we can get would be greatly appreciated.
Safety Policy: Pangaea Biosciences is committed to maintaining a safe environment for its staff and animal subjects. We are also dedicated to minimizing the impact of its operations on the environment surrounding our research sites. It is the policy of Pangaea Biosciences to conduct its operations in conformance with applicable laws, regulations, and relevant published standards and practices for health, safety, and environmental protection.
Notes
(1) Each group and each enclosure will contain the same number of breeding pairs. Subsequent offspring will be paired randomly.
(2) We may seek a more cost effective method. Another option is Single Pass DNA Sequencing.
References
(2013). Advantages of the mouse as a model organism. The Jackson Institute. Retrieved from http://research.jax.org/
(2013). Background Radiation. Science Clarified. Retrieved from http://www.scienceclarified.com/
(2013). Evolutionary and Experimental Biology of Ageing. Max Planck Institute for Biology of Ageing. Retrieved from http://www.age.mpg.de/
(2013). Fact Sheet on Biological Effects of Radiation. United Sates Nuclear Regulatory Commission. Retrieved from http://www.nrc.gov/
(2013). Radiation in Everyday Life. International Atomic Energy Agency. Retrieved from http://www.iaea.org/
Abrahamson, S., BENDER, M. A., CONGER, A. D., & WOLFF, S. (1973). Uniformity of radiation-induced mutation rates among different species.
Beeson, Steven; Mayer, James W. "12.2.2 Discoveries beyond the visible". Patterns of light: chasing the spectrum from Aristotle to LEDs. New York: Springer. p. 149. ISBN 978-0-387-75107-8.
Carroll, S. B., Grenier, J., & Weatherbee, S. (2009). From DNA to diversity: molecular genetics and the evolution of animal design.
Cockell, C., & Blaustein, A. R. (Eds.). (2001). Ecosystems, evolution, and ultraviolet radiation. Springer.
Damkaer, D. M. (1982). Possible influences of solar UV radiation in the evolution of marine zooplankton. In The role of solar ultraviolet radiation in marine ecosystems (pp. 701-706). Springer US.
De Gruijl, F. R. (1999). Skin cancer and solar UV radiation. European Journal of Cancer, 35(14), 2003-2009.
Dubrova, Y. E. (2009). Radiation-induced mutation at tandem repeat DNA loci in the mouse germline: spectra and doubling doses.
Green, E. L., & Roderick, T. H. (1966). Radiation genetics. Biology of the laboratory mouse. New York: McGraw-Hill, 87-150.
Hessen, D. O. (2008). Solar radiation and the evolution of life. Solar Radiation and Human Health, 123-136.
Houldsworth, J., & Lavin, M. F. (1980). Effect of ionizing radiation on DNA synthesis in ataxia telangiectasia cells. Nucleic acids research, 8(16), 3709-3720.
James H. Sang (2001-06-23). "Drosophila melanogaster: The Fruit Fly". In Eric C. R. Reeve. Encyclopedia of genetics. USA: Fitzroy Dearborn Publishers, I. p. 157. ISBN 978-1-884964-34-3.
Lage, K., Karlberg, E. O., Størling, Z. M., Olason, P. I., Pedersen, A. G., Rigina, O., ... & Brunak, S. (2007). A human phenome-interactome network of protein complexes implicated in genetic disorders. Nature biotechnology, 25(3), 309-316.
López‐Bigas, N., & Ouzounis, C. A. (2004). Genome‐wide identification of genes likely to be involved in human genetic disease. Nucleic acids research, 32(10), 3108-3114.
Olive, P. L., Banáth, J. P., & Durand, R. E. (1990). Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the" comet" assay. Radiation research, 122(1), 86-94.
Ostling, O., & Johanson, K. J. (1984). Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochemical and biophysical research communications, 123(1), 291-298.
Paul, N. D., & Gwynn-Jones, D. (2003). Ecological roles of solar UV radiation: towards an integrated approach. Trends in Ecology & Evolution, 18(1), 48-55.
Ravanat, J. L., Douki, T., & Cadet, J. (2001). Direct and indirect effects of UV radiation on DNA and its components. Journal of Photochemistry and Photobiology B: Biology, 63(1), 88-102.
Rothschild, L. J. (1999). The influence of UV radiation on protistan evolution. Journal of Eukaryotic Microbiology, 46(5), 548-555.
Rozema, J., Björn, L. O., Bornman, J. F., Gaberščik, A., Häder, D. P., Trošt, T., ... & Meijkamp, B. B. (2002). The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 66(1), 2-12.
Teoule, R. (1987). Radiation-induced DNA damage and its repair. International Journal of Radiation Biology, 51(4), 573-589.
Thacker, J., & Cox, R. O. G. E. R. (1975). Mutation induction and inactivation in mammalian cells exposed to ionising radiation.
Todd, P. (1994). Cosmic radiation and evolution of life on Earth: roles of environment, adaptation and selection. Advances in Space Research, 14(10), 305-313.
Tusher, V. G., Tibshirani, R., & Chu, G. (2001). Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences, 98(9), 5116-5121.
Yauk, C. L., Dubrova, Y. E., Grant, G. R., & Jeffreys, A. J. (2002). A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 500(1), 147-156.