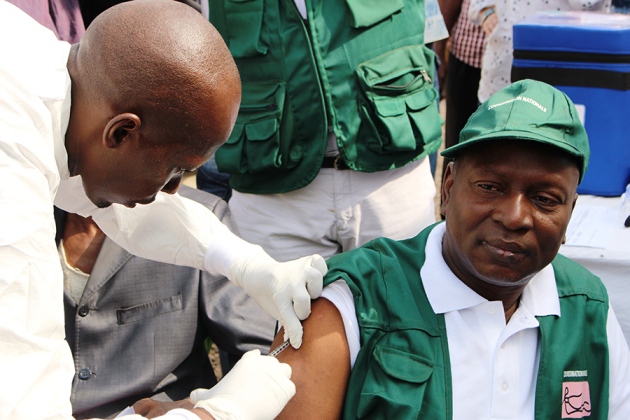
An experimental Ebola vaccine seems to confer total protection against infection in patients at high risk of contracting the virus, according to preliminary results of a trial in Guinea that were announced today and published in The Lancet. They are the first evidence of any kind that a vaccine protects humans from Ebola infection.
“We believe the world is on the verge of an efficacious Ebola vaccine," Marie-Paule Kieny, the World Health Organization's assistant director-general for health systems and innovation, said during a press conference in Geneva, Switzerland, today.
The results also have implications for outbreak response in general. "This is illustrating that it is feasible to develop vaccines much faster than we’ve been doing," says Adrian Hill, a vaccine scientist at the University of Oxford, UK, who is involved in testing a different Ebola vaccine. “We just need to go on and develop them and get on with them before outbreaks appear.”
An estimated 11,280 people have died during the current West African Ebola epidemic, according to WHO data as of 30 July.
Ring strategy
The Guinea trial — called ‘Ebola, ça suffit' (‘Ebola, that's enough’, in French) — tested a ring vaccination design, a strategy that was borrowed from successful smallpox eradication efforts in the 1970s. After one patient contracts the disease, their close contacts are vaccinated in hopes of stemming the virus' onward spread.
The Guinea trial included two arms: one in which adult contacts and their contacts were vaccinated shortly after the first patient developed Ebola, and a second in which contacts instead received the vaccine three weeks later2. The trial tested a vaccine composed of an attenuated livestock virus engineered to produce an Ebola protein, called rVSV-ZEBOV. The vaccine was developed by Public Health Agency of Canada and then licensed to the companies NewLink Genetics and Merck.
Of the 2,014 people who received the vaccine immediately as part of the first arm, none developed Ebola ten days after getting the vaccine, a window that allows for the vaccine to summon an immune response and accounts for pre-existing infection. Several, however, did develop the disease during that window. That result compares with 16 infections among 2,380 people who got the vaccine 3 weeks later. The findings mean the vaccine provided 100% protection from the virus, though the study's small size means that the vaccine’s true protection rate may be slightly lower, Kieny says. The authors of the paper put its true effectiveness at between 75% and 100%.
Hill describes the results as “excellent". "This vaccine works very well for three weeks. That’s good news for an outbreak situation," he says. However, it remains to be seen how long the protection against Ebola lasts. "Will it work at six months? This trial doesn’t tell us that. That’s the next stage," he says.
Immediate vaccination
The results come from data up to 3 July, but Kieny says no new infections have since been detected in people who got the vaccine immediately. Based on these results, she said the delayed vaccination arm would be ended, and all contacts would receive the vaccine immediately. Additionally, adolescents and children are now likely to receive the vaccine.
Guinea's outbreak is waning — just 4 cases were detected in Guinea the week of 26 July — but Kieny said the rVSV-ZEBOV should help bring the outbreak to an end. “We will continue as long as there are cases,” she said.
Health officials began vaccinating patients in Guinea in April 2015, when dozens of patients there were still being diagnosed each week. At that time, Ebola was all but gone from Liberia and cases were down drastically in Sierra Leone, the two West African nations where different Ebola vaccine trials were being planned.
With case numbers waning, many experts saw the Guinea trial as the best hope of determining whether an effective Ebola vaccine was even a possibility. The ring vaccination design it used was more likely to return a statistically meaningful result, compared with other trial designs now being conducted in Liberia and Sierra Leone, because the patients enrolled in the trial were at a very high risk of infection.
“A vaccine that didn’t exist in the clinic a year ago has shown not only to be effective in a phase III trial but to control an outbreak, which is a fantastic result," says Hill. (Phase III is the stage of a clinical trial which determines whether a vaccine works and is generally required for regulatory approval).
“This trial dared to use a highly innovative and pragmatic design, which allowed the team in Guinea to assess this vaccine in the middle of an epidemic," said Jeremy Farrar, director of the Wellcome Trust in London, which helped to fund the trial, in a statement. "Our hope is that this vaccine will now help bring this epidemic to an end and be available for the inevitable future Ebola epidemics," he said.
Source: Nature, Full Article